WHMIS – What You Need To Know

The Workplace Hazardous Materials Information System (WHMIS), Canada’s national hazard communication standard, is changing to incorporate the Globally Harmonized System of Classification and Labelling of Chemicals (GHS) – an internationally recognized standard for hazard classification and communication.

Why is this important? MSDS to SDS
By May 31st 2018, we will replace our current MSDS with a multi-page 16 section Safety Data Sheet (SDS). The new SDS format will contain enhanced technical environmental, health, safety and regulatory information. Product labels as well as workplace labels will also be changing to comply with the new regulations. This document is intended to give you an overview of the changes to come.

Why is GHS being implemented?
Suppliers, employers and employees face many challenges due to the lack of international alignment in the classification, labelling and provision of safety information for workplace hazardous chemicals. This situation may present a problem for global trade as well as causing risk for workers due to inconsistent, confusing information.
For example, before GHS, a product with an Oral LD50 of 257 mg/kg could have been classified as:
- Toxic in Canada, US, Japan and Korea
- Harmful in European Union, Australia, Malaysia and Thailand
- Moderately toxic in China
- Hazardous in New Zealand
- Non-toxic in India
What is GHS?
GHS stands for Globally Harmonized System of Classification and Labelling of Chemicals. GHS is a system that defines and classifies the hazards of chemical products, and communicates health and safety information on labels and material safety data sheets (called Safety Data Sheets, or SDSs, in GHS). The goal is that the same set of rules for classifying hazards, and the same format and content for labels and safety data sheets (SDS) will be adopted and used around the world.
How is this going to impact us?
On February 11, 2015, the Government of Canada published in the Canada Gazette, Part II the Hazardous Products Regulations (HPR). The new WHMIS, now called “WHMIS 2015”, is based on the new requirements contained in the Hazardous Products Regulations (HPR) and Hazardous Products Act (HPA), as amended in 2014. GHS will not replace WHMIS. GHS will, however, create some important changes to WHMIS. While WHMIS 2015 includes new harmonized criteria for hazard classication and requirements for labels and safety data sheets (SDS), the roles and responsibilities for suppliers, employers and workers have not changed. GHS brings the following changes:
- New classification rules and hazard classes
- A standardized format for Safety Data Sheet (formerly MSDS) that will now contain 16 sections instead of 9
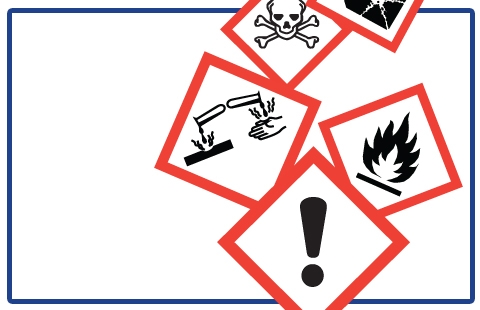
- New hazard pictograms
- New label requirements
How will GHS change WHMIS?
Standardized Pictograms in WHMIS 2015
Labels will use new standardized GHS pictograms as shown below.

Safety Data Sheet
Material Safety Data Sheets (MSDS) will be referred to as a Safety Data Sheet (SDS). The GHS standardizes the formatting of SDSs into a MSDS strict 16 section document with a specific order:
- Identification
- Hazard Identification
- Composition/information on ingredients
- First-aid measures
- Fire-fighting measures
- Accidental release measures
- Handling and Storage
- Exposure controls/personal protection
- Physical and chemical properties
- Stability and reactivity
- Toxicological information
- Ecological information
- Disposal considerations
- Transport information
- Regulatory information
- Other information
Hazard Ratings
New GHS rating are inverted and are only found on safety data sheets (SDSs). Some hazard classes may only have 4 categories
Timeline for Transition Period
A multi-year transition plan has been announced. From now until May 31, 2018, suppliers can use either WHMIS 1988 or WHMIS 2015 to classify and communicate the hazards of their products (the suppliers must use one system or the other). Beginning June 1st, 2018, manufacturers must discontinue old labels/MSDS. Dustbane Products Ltd. will transition to WHMIS 2015 by May 31st, 2018.
PHASE 1:
February 11, 2015 to May 31, 2018
Introduction of new labels and SDSs
PHASE 2a:
June 1, 2018 to September 1, 2018
End of sales with old labels/MSDS by manufacturer
PHASE 2b:
June 1, 2018 to September 1, 2018
End of sales/import/receipt of products with old labels/MSDS
PHASE 3:
June 1, 2018 to November 30, 2018
Full implementation